USANA Quality Guarantee
USANA Health Sciences offers the highest quality products you can find anywhere. To guarantee their quality, USANA products are manufactured under rigorous conditions, and each product goes through four major areas of testing: Purity, Identity, Composition, and Strength. This ensures that you can be confident in USANA’s products, and that what’s on the label is in the product.
The quality control process is unique for each product and each raw material. USANA’s Research and Development team custom designs the testing necessary for each individual product and ingredients. Hepasil DTX is just one of USANA’s many high-quality products. Below you will find some of what goes into guaranteeing the quality of this product.
Hepasil DTX Testing for Purity, Identity, Composition, and Strength
| Item Tested | Purity | Identity | Composition | Strength | Strength Dosage | Third-Party Validated |
| Hepasil DTX Tablet | ✅ | ✅ | ✅ | ✅ | ✅ | ✅ |
| Biotin | ✅ | ✅ | ✅ | ✅ | 75 µg | ✅ |
| Choline | ✅ | ✅ | ✅ | ✅ | 51.3 mg | ✅ |
| Milk Thistle Extract | ✅ | ✅ | ✅ | ✅ | 80 mg | ✅ |
| N-Acetyl L-Cysteine | ✅ | ✅ | ✅ | ✅ | 75 mg | ✅ |
| Alpha-Lipoic Acid | ✅ | ✅ | ✅ | ✅ | 67 mg | ✅ |
| Broccoli Concentrate | ✅ | ✅ | ✅ | ✅ | 25 mg | ✅ |
| Inositol | ✅ | ✅ | ✅ | ✅ | 25 mg | ✅ |
| Green Tea Extract | ✅ | ✅ | ✅ | ✅ | 15 mg | ✅ |
| Olivol | ✅ | ✅ | ✅ | ✅ | 15 mg | ✅ |
| Meriva Curcumin | ✅ | ✅ | ✅ | ✅ | 1.33 mg | ✅ |
Purity
Supplements are designed to nourish your body and support your health, not harm it. Just how food can become contaminated without proper food safety, ingredients in your supplement can be contaminated too. Potential contamination becomes especially critical in supplement raw materials. That’s because extraction and concentration of beneficial components can concentrate the harmful ones too.
Testing of raw ingredients and the finished product is essential to make sure that you won’t be harmed by biological contaminants, heavy metals, pesticides, and other forms of contamination.
Hepasil DTX Biological Contamination Testing Specifications
Total plate count: ≤ 3000 CFU/gram (10,000 CFU/gram milk thistle)
Escherichia coli: Absent in 10 g sample
Salmonella species: Absent in 10 g sample
Staphylococcus aureus: Absent in 10 g sample
Total molds and yeast: ≤ 300 CFU/gram
Hepasil DTX Chemical Contamination Testing Specifications
Lead: ≤ 1.71 ppm
Arsenic: ≤ 5.15 ppm
Mercury: ≤ 5.15 ppm
Cadmium: ≤ 1.71 ppm
Solvents Tested: Acetonitrile, Benzene, Carbon tetrachloride, Chlorobenzene, Chloroform, Cumene, Cyclohexane, 1,2-Dichloroethane, 1,1-Dichloroethene, 1,2-Dichloroethene, 1,2-Dimethoxyethane, N,N-Dimethylacetamide, N,N-Dimethylformamide, 1,4-Dioxane, 2-Ethoxyethanol, Ethylene glycol, Formamide, Hexane, Methanol, 2-Methoxyethanol, Methylbutylketone, Methylcyclohexane, Methylene chloride, Methylisobutylketone, N-Methylpyrrolidone, Nitromethane, Pyridine, Sulfolane, 1,1,1-Trichloroethane
Residual Solvents Test Specifications: Tested to conform with USP standards for each individual solvent.
Pesticides Tested: Acephate/Orthene, Alachlor, Alachlor, Aldrin, Azinphos ethyl, Azinphos methyl/Gluthion, BHC Total, BHC Alpha, BHC Beta, BHC, delta, BHC gamma/Lindane, Bromophos ethyl, Bromophos methyl, Bromopropylate/Acarol, Chlordane (total), Chlordane alpha, Chlordane gamma, Chlordane Oxy, Chlorfenvinphos, Chlorpyrifos/Dursban, Chlorpyrifos Methyl, Chlorthal-Dimethyl/Dacthal, Cyfluthrin (Sum), Cyhalothrin Lambda, Cypermethrin (sum of isomers), DDD-2,4’/DDD-o,p’, DDD-4,4’/DDD-p,p’, DDE-2,4’/DDE-o,p’, DDE-4,4’/DDE-p,p’, DDT, Total, DDT-2,4’/DDT-o,p’, DDT-4,4’/DDT-p,p’, Deltamethrin, Diazinon, Dichlofluanid, Dichlorvos/DDVP, Dicofol-o,p’, Dieldrin, Dimethoate/Cygon, Dithiocarbamates (CS2), Endosulfan (total), Endosulfan I, Endosulfan II, Endosulfan sulfate, Endrin aldehyde, Ethion, Endrin ketone, Ethion, Etrimfos/Etrimophos, Fenchlorphos/Ronnel, Fenchlorphos-oxon, Fenitrothion, Fenpropathrin, Fensulfothion (Sum), Fensulfothion-oxon, Fensulfothion-oxon sulfone, Fensulfothion-sulfone, Fenthion/Baytex, Fenthion sulfone, Fenthion sulfoxide, Fenthion-oxon, Fenthion-oxon sulfone, Fenthion-oxon sulfoxide, Fenvalerate/Sanmarton, Ferbam, Flucythrinate, Fluvalinate, tau, Fonofos/Dyfonate, Heptachlor, Heptachlor epoxide, Heptachlor epoxide A, Heptachlor epoxide B, Hexachlorobenzene/HCB, Malaoxon/Malathion OA, Malathion, Mancozeb, Maneb, Mecarbam, Methacrifos, Methamidophos, Methidathion/Supracide, Methoxychlor, Mirex, Monocrotophos/Azodrin, N-Desethyl-Pirimiphos-methyl, Nabam, Omethoate, Parathion, Parathion Methyl/Methyl Parathion, Parathion Methyl Oxon, Parathion Oxon/Paraoxon, Pendimethalin/Prowl, Pentachloroaniline, Pentachloroanisol/PCA, Pentachloronitrobenzene/PCNB/Quintozene, Pentachlorothioanisole/Methyl pentachlorophenyl sulfide/MPCPS, Permethrin/Pounce, Permethrin, cis, Permethrin, trans, Phosalone/Zolone, Phosmet/Imidan, Piperonyl Butoxide, Pirimiphos ethyl, Pirimiphos methyl ,Procymidone, Profenophos/Curacron, Propineb, Prothiofos/Tokuthion, Pyrethrin/Pyrethrum, Pyrethrin I, Pyrethrin II, Quinalphos, Thiram, Zineb/Metiram, Ziram
Pesticides Test Specifications: Tested to conform with USP standards for each individual pesticide.
Identity
Identifying the beneficial components of the finished good and each raw material is the next step of the quality control process.
Following manufacturing, every batch is reviewed and approved to meet good manufacturing practices and product specifications for each raw material and the finished good. The identity for each raw material within the product must meet the identity specified on the product label.
For raw materials, each batch of raw material is sampled, inspected, and analytically tested. They’re tested to ensure that the raw material contains the desired nutrients in the right form and concentration.
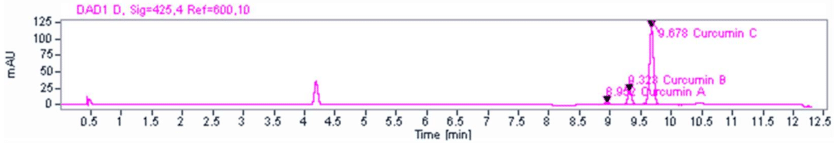
Each raw material has its own specific testing procedure and method. An overview of the test method and a sample method for Meriva Curcumin can be found below.
| Ingredient | Test Method |
| Biotin | LCMS |
| Choline | LCMS |
| Milk Thistle Extract | HPTLC and HPLC |
| N-Acetyl L-Cysteine | HPLC and FTIR |
| Alpha-Lipoic Acid | HPLC |
| Broccoli Concentrate | FTIR |
| Inositol | LCMS |
| Green Tea Extract | HPTLC |
| Olivol | HPTLC |
| Meriva Curcumin | HPLC |
LCMS = Liquid Chromatography Mass Spectroscopy
HPTLC = High Performance Thin Layer Chromatography
HPLC = High Pressure Liquid Chromatography
FTIR = Fourier Transform Infrared Spectroscopy
Test Methodology Example: Meriva Curcumin
- Prepare 0.03% formic acid
- Prepare solvent solution MeOH:DMSO (75%:25%)
- Prepare HPLC
- Install HPLC column
- Purge with formic acid
- Turn on detector and start flow with 65% formic acid and 35% methanol.
- Prepare standard sample by mixing reference curcumin and solvent (from step 2)
- Prepare raw material sample by mixing sample curcumin with solvent (from step 2)
- Load vials into HPLC sampler and start run. Total run time is 12.3 minutes.
- Collect data and calculate results.
Example HPLC Output
Composition
Each ingredient has its own composition that is tested and verified by USANA before it can be used in manufacturing. For example, milk thistle has a huge number of different components in the plant, and those could be found in a variety of different proportions within a raw material. Silymarin is the primary component of interest by USANA scientists when they designed this product. So, the milk thistle used in Hepasil DTX is verified to have a composition of 80% silymarin.
The milk thistle extract is also reviewed for other beneficial components, source location, and potential allergens. A similar analysis is performed for every raw material used in Hepasil DTX.
Tablet composition is important to make sure that your body can digest the tablet, and that individual nutrients reach and benefit your cells. This starts with the research and development process, where the product is formulated. The active ingredients aren’t the only important part of a tablet. Additional ingredients must be added to make everything form into a solid and durable tablet, but also one that can be easily broken down in the body.
After the right combination of ingredients is determined, the tablet can finally go into production. Every finished batch of product is analyzed and tested for weight, thickness, disintegration, friability (tendency to break), and hardness.
Hepasil DTX Composition
Appearance: Hepasil DTX is a coated Bi-Layer tablet. One layer is white to off white; the other layer is tan to beige. Some color variation is normal.
Weight: 980 mg
Thickness: 6.6 mm
Disintegration: 100% disintegration in ≤ 20 minutes
Strength
Finally, finished products are tested to have the strength and potency guaranteed on the labels—one of the main reasons why people trust their health to USANA. This requires repeating many of the purity tests from the individual tests from the identification step above. Adjustments are made to the methods as needed, due to differences between a finished good and raw materials.
Stability testing is also performed to guarantee the potency of the product throughout shelf-life.
Long-Term Product Stability
Vitamins, plant compounds, and other nutrients can oxidize and degrade over time. Over time, this can result in the level of these nutrients being lower than the amount listed on the level. However, a properly formulated product helps to counteract this risk and guarantee the viability of the product throughout its shelf-life. This includes meeting at least 100% label claim throughout shelf-life.
Long-term stability testing is necessary to test product formulation. This ensures that there are no significant physical, chemical, or microbiological changes to the Hepasil DTX Bi-Layer tablet that would negatively impact its beneficial properties for the consumer.
Stability studies are conducted on Hepasil DTX following guidelines set forth by the U.S. FDA, Canada NHPD, Australia’s TGA, and the ICH. The product is tested for potency and purity at the date of manufacture, then samples are placed in a controlled test environment. The test environment is maintained at 25 ± 2 °C and 60 ± 5% relative humidity throughout the shelf-life of the product (24 months from the date of manufacture).
Samples are pulled from the stability batch at 12 and 24 months. Potency of the active ingredients is tested at both of these intervals, and the 24-month sample is tested for biological contamination.
Government and Third-Party Validation
A tremendous amount of research goes into formulating, manufacturing, and testing each USANA product. You don’t have to take our word for it though. USANA facilities and manufacturing processes are regularly audited by outside organizations, finished products have a variety of certifications, and products are studied in collaboration with research organizations around the world.
- FDA
- TGA
- Other Global Government Entities
- USP
- NSF
- Consumer Lab
- Informed Choice
- OK Kosher
- IFANCA Halal